Pioneering Research & Production

Rigorous Scientific Research
Stallen conducts rigorous research and development before launching any product, including in-vitro, in-vivo, and field trials tailored to real-world veterinary needs. Our commitment to science includes publishing findings, running multi-year testing programs, and continuously improving product quality through data-driven insights.
Clinical Trials
Field Trials
We conduct controlled field trials on farms and commercial operations to assess the real-world performance of our feed additives and medicines under actual stress conditions such as disease pressure, environmental variation, and management practices. The data generated supports product validation, is peer-reviewed or published where applicable, and serves as credible proof for technical marketing and customer education.
Academic Trials
In collaboration with universities, veterinary colleges, and research institutes, we design and execute academic trials that explore product efficacy, pharmacokinetics, and comparative performance against existing treatments. These partnerships help us build a strong scientific foundation, generate publishable data, and strengthen the regulatory and technical positioning of each product.
In-vitro Testing
For microbiological, immunological, and antimicrobial products, including vaccine candidates and disinfectants, we perform comprehensive in-vitro testing using standardized strains and clinical isolates. These assays measure efficacy against specific pathogens, including bacterial, viral, and fungal organisms, offering early-phase validation and insights into mode of action, spectrum, and resistance potential.

Certificates & Approvals
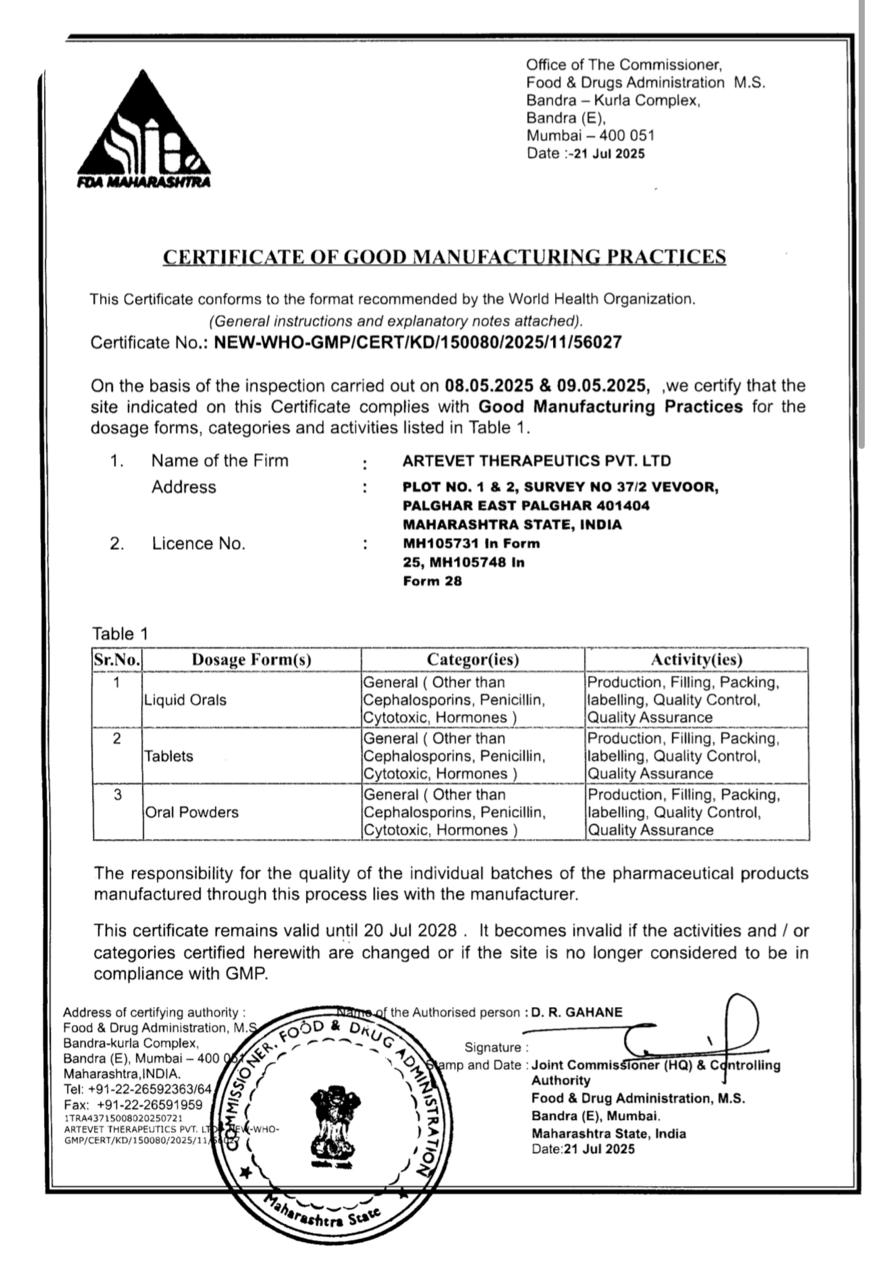
WHO-GMP
Our pharmaceutical products are produced in WHO-GMP approved plants, following strict international standards for safety and efficacy.
2024
ISO 9001
All our feed additives are manufactured in ISO 9001:2015 certified facilities, ensuring consistent quality management across every batch.
2024

EU-GMP
All vaccines are sourced from EU-GMP certified facilities, meeting stringent European regulatory and manufacturing requirements.
2024

FDA
Our APIs and finished medicines are approved by the Indian FDA, and we hold all relevant drug manufacturing and marketing licenses.
2024

Patents and Innovations

Novel Early Chick Nutrition Product
Stallen’s Survinova is India’s first and leading product for day old chick nutrition and hydration. It is used by over 18 million chicks annually.
MS Killed Vaccine
We are proud to work with Fatro’s MS-VAC – India’s only mycoplasma synoviae killed vaccine – trusted by over 4 million birds ever year!
Unique Salmonella Vaccine
Fatro’s BIO-VAC SGP 695 is a patented product and the world’s only drinking water based vaccine for salmonella gallinarium/pullorum.
No. 1 Indian Water Disinfectant
Cleantab is India’s highest selling water disinfectant solution – we are treating 11.5 billion liters annually. Selling 52 tablets every minute.
Explore our Production Hub
Manufacturing Operations
From API to finished formulations, Stallen’s seamlessly integrated global production network ensures total control and uncompromised quality at every step.
2
Countries
5
Locations




