Salmonella is a leading cause of foodborne illness globally, with non-typhoidal serotypes like S. Enteritidis and S. Typhimurium responsible for the majority of gastroenteritis cases. In India, though national surveillance data is limited, reports under the Integrated Disease Surveillance Programme (IDSP) show hundreds of foodborne outbreaks annually, with thousands affected. Vulnerable groups such as infants and immune compromised individuals are particularly at risk, as even low doses of Salmonella can trigger severe illness. South Asia reports an estimated 2.2 cases of invasive non-typhoidal Salmonella per 100,000 people annually, with a high fatality rate. Poultry—especially chicken meat and eggs—remains a primary reservoir, with multiple studies indicating widespread contamination and rising antimicrobial resistance, posing significant public health risks.
With increasing demand for poultry and growing concerns over antibiotic resistance, India faces the challenge of balancing production efficiency with food safety. Regulatory moves to restrict antibiotic use, combined with consumer preference for antibiotic-free meat, are pushing the industry towards alternative control strategies. Preharvest measures like biosecurity, improved farm hygiene, and feed additives, along with postharvest controls such as HACCP implementation, are being emphasized. Vaccination has become a core preventive tool; however, the need persists for more robust vaccines offering cross-protection against diverse and emerging serotypes. This review highlights the current understanding of Salmonella in poultry and explores sustainable control approaches suitable for the Indian context.
Transmission:
Salmonella primarily spreads through the faeces of infected chicks, contaminated feed, water, and litter. Human activities, such as farm visits without proper biosecurity and movement between chicken houses, also contribute to its spread. Transmission occurs via direct contact with infected birds or indirectly through contaminated environments. Vertical transmission, particularly through infected eggs, is key in sustaining outbreaks, as asymptomatic carriers can pass the bacteria to offspring for up to 14 weeks. These bacteria can survive in the environment for months under favourable conditions, though sunlight and high temperatures reduce their persistence. Wild birds, mammals, and insects, especially red mites, can act as vectors, complicating control efforts.
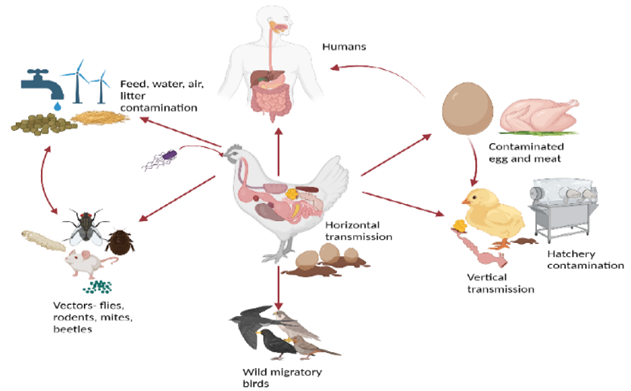
Fig.1: Transmission of Salmonella.
Pathogenesis:
Salmonella pathogenesis starts when bacteria are ingested, surviving the stomach’s acidity to invade the intestinal mucosa using virulence factors like plasmids, toxins, fimbriae and flagella. They infect non-phagocytic cells and macrophages, triggering inflammation and evading the immune system. The bacteria spread via the bloodstream to organs like the liver, spleen and kidneys causing symptoms such as diarrhoea, loss of appetite and depression, leading to high mortality, especially in young chicks. Salmonella can be transmitted both vertically and horizontally. It induces inflammation, macrophage apoptosis, and can cause severe haemolytic anaemia, leading to rapid death. The incubation period is typically 4 to 6 days.
Clinical signs:
Pullorum disease mainly affects young birds particularly chicks under 3-4 weeks old, with peak mortality at 2-3 weeks. Infected embryos may die in the egg and recently hatched chicks often exhibit signs of acute septicaemia such as depression, weakness, loss of appetite, drooping wings, huddling, laboured breathing, dehydration, and ruffled feathers. White, viscous diarrhoea and faecal pasting around the vent are common. Older chicks may experience a less acute disease course, sometimes developing arthritis or blindness. Survivors may be underweight, poorly feathered and less productive as adults. Infections in birds older than 4 weeks are usually asymptomatic but can result in decreased egg production and fertility. Fowl typhoid affects birds of all ages with symptoms like depression, appetite loss, weight loss, dehydration, ruffled feathers, yellowish diarrhoea and respiratory distress. Older birds may experience decreased egg production, fertility, and hatchability leading to anaemia with pale, shrunken combs and wattles. Atypical outbreaks, such as one in quail characterized by decreased egg laying and high mortality without clear clinical signs can also occur.
Diagnosis:
Lesions may be highly suggestive; however, diagnosis should be confirmed by isolation, identification, and serotyping of S Pullorum. Infections in mature birds can be identified by serological tests, followed by necropsy evaluation complemented by microbiological culture and typing for confirmation.
Official testing recommendations for flocks in the US are outlined in the National Poultry Improvement Plan (NPIP). The NPIP lists approved rapid assays for Salmonella. These include, for example, PCR assay and lateral flow immunoassays. Some assays are for the general detection of all Salmonella spp. Further typing is required after these general detection assays. Other NPIP-approved rapid assays are specific for Salmonella enterica serotype enteritidis like plasmid profiling and ribotyping, aid in accurate identification and differentiation.
Postmortem lesions:
The liver is yellowish in colour with haemorrhagic streaks. In chronic cases the ovary consists of pedunculate and misshapen ovules. The most obvious lesion includes enlarged and congested liver, which becomes dark red or brown (bile-stained liver) after exposure to the atmosphere. There may be multiple necrotic areas throughout the liver. There is congestion and necrosis of the liver and spleen with catarrhal enteritis.

Fig. 2 Granulomatous hepatitis, liver, chicken
Antimicrobial Resistance:
Antimicrobial resistance is a growing global challenge, worsened by insufficient assessments of Salmonella resistance and lack of regulation. The easy access to antimicrobials without prescriptions, along with incomplete treatments, exacerbates the problem. In poultry farming, the overuse of antibiotics has led to the development of resistant strains, including those producing extended spectrum beta-lactamases (ESBLs), posing a serious threat to both public health and the poultry industry (Parvej et al., 2016). Resistance mechanisms include bacterial target modifications, changes in cell membrane permeability, and efflux pumps. Misuse of antibiotics has resulted in the rise of multidrug-resistant Salmonella strains, making treatment more difficult and highlighting the need for more careful antibiotic use (Farhat et al., 2023).
Prevention and control:
Preventing and controlling salmonellosis on poultry farms is essential. Key strategies include removing infected birds, keeping healthy and sick birds separate and using testing methods like tube-agglutination to screen flocks. Strong biosecurity measures, such as strict hygiene, controlled farm access, and proper management of litter, feed, and water, help reduce disease spread. Without these measures, fowl typhoid poses a significant economic threat, highlighting the need for organized control programs with accurate testing and prompt action.
Vaccination plays a crucial role in preventing and controlling salmonellosis on poultry farms. Effective vaccines can help reduce infection rates of fowl typhoidand salmonella enteritidis providing long-term protection for flocks. In addition to vaccination, strategies like early identification and removal of infected birds, routine testing, and strict biosecurity measures (e.g., hygiene, controlled farm access) are essential for minimizing disease spread. Combining vaccination with proper management of litter, feed, and water enhances flock health and reduces the economic impact of fowl typhoid, making it a key component of any comprehensive disease control program.
Stallen South Asia Pvt. Ltd. is offering a unique live vaccine BIO-VAC SGP 695, against fowl typhoid and salmonella Enteritidis.
Key features of BIO-VAC SGP 695
- BIO-VAC SGP 695 contains the live attenuated strain SGP 695 AV of Salmonella gallinarum/pullorum that induces a strong active immunity in vaccinated pullets, against fowl typhoid, reducing mortality, clinical signs, pathological lesions, losses in eggs production and against Salmonella enteritidis infection, reducing the colonisation of internal organs and ovary.
- In drinking water administration.
- Stable attenuated and total apathogenicity of the vaccine strain.
- Reduction of vaccination procedure costs.
Why choose BIO-VAC SGP 695 than SG 9R salmonella vaccine?
| BIO- VAC SGP 695 | SG 9R vaccine | |
| Strain | 695 AV (Live attenuated) | 9R (Rough strain with possible reversion) |
| Characteristics | Does not revert to virulence | Possible reversion to virulence |
| Targeted Infections | Salmonella gallinarum, Salmonella pullorum, Salmonella Enteritidis | Primarily Salmonella gallinarum (Fowl Typhoid) |
| Administration | Oral (via drinking water) | Subcutaneous injection |
| Vaccination Program | Initial dose at 6-8 weeks, second at 16-18 weeks. Early dose if early infection history. | Initial dose at 6 weeks, revaccination every 12 weeks for layers. |
| Effectiveness | Broad protection including Salmonella Enteritidis | Focused on protection against Salmonella gallinarum |
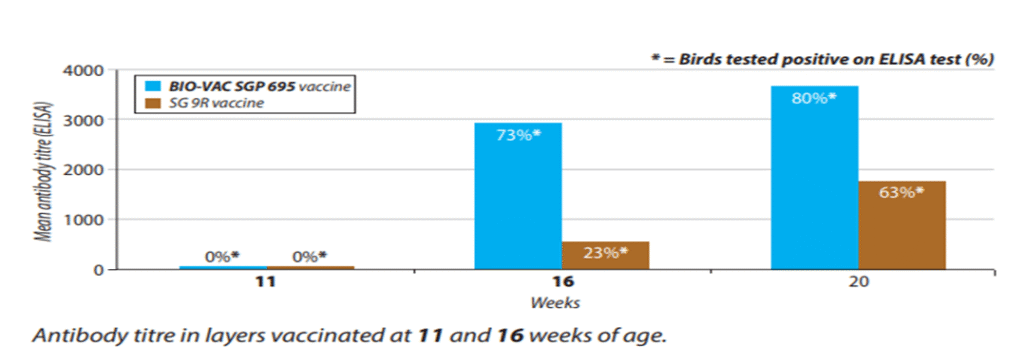
Fig 3: ELISA report of BIO-VAC SGP 695 and SG 9R vaccine

Dr. Kishor Gedam
Product Manager- Therapeutics
Stallen South Asia Pvt. Ltd

Dr. Sanjay Singhal
Chief Operating Officer Stallen South Asia Pvt. Ltd
References
- Jajere, S.M., 2019. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World, 12: 2231-0916.
- Kebede, D., Z. Tekle, A. Gezahegne, G. Abdisa, R. Mulatu, A. Wondimu and A. Ayesheshum, 2019. Review on Salmonella Gallinarum-Pullorum. British Journal of Poultry Sciences, 8(1): 10-16.
- Davison, S., 2019. Pullorum Disease in Poultry, Laboratory of Avian Medicine and Pathology, School of Veterinary Medicine, University of Pennsylvania, pp: 1-20.
- Parvej, M.S., K.H. Nazir, M.B. Rahman, M. Jahan,M.F. Khan and M. Rahman, 2016. Prevalence and characterization of multi-drug resistant Enterica serovar gallinarum biovar Pullorum and gallinarum from chicken. Vet. World, 9(1): 65-70.
- Farhat, M., Khayi, S., Berrada, J., Mouahid, M., Ameur, N., El-Adawy, H., & Fellahi, S. (2023). Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum in Poultry: Review of Pathogenesis, Antibiotic Resistance, Diagnosis and Control in the Genomic Era. Antibiotics, 13(1), 23.