Dr. Amit V. Janbandhu, Dr. Sanjay Singhal
Abstract
This study evaluates the efficacy of PEPIGRO, a Bacillus licheniformis-based probiotic and postbiotics as antimicrobial peptide (AMPs), on the growth and health performance of commercial broilers under field conditions. A total of 36,000 straight-run broiler chicks were assigned to control and treatment groups, with the latter receiving PEPIGRO supplementation at 300 g/ton of feed. The trial was conducted over 42 days during extreme heat (42–45°C), and assessed body weight, feed intake, feed conversion ratio (FCR), weekly gain, and mortality. PEPIGRO supplementation resulted in an 8.18% increase in body weight, a 6.59% rise in feed intake, and a 6.22% improvement in weekly gain compared to the control, alongside a 1.68% enhancement in FCR. Mortality was notably reduced by 28.08%, indicating improved survivability. These findings demonstrate that dietary inclusion of PEPIGRO effectively enhances broiler growth performance, feed efficiency, and health, supporting the role of Bacillus licheniformis as a promising antibiotic alternative under commercial field stressors.
Introduction
In recent decades, antibiotics have been extensively used in animal husbandry for their dual purposes of promoting growth and preventing or treating bacterial infections. However, the excessive and prolonged use of these drugs has led to serious public health and environmental concerns, including the emergence of antibiotic-resistant pathogens and environmental contamination, which pose risks to both humans and animals (Tang et al., 2017). Consequently, international authorities implemented strict prohibitions on antibiotic growth promoters (Organization, 1999), prompting researchers to explore alternative, safer strategies to enhance livestock productivity.
Among the promising alternatives, several bioactive feed additives—such as probiotics (Xu et al., 2021), antimicrobial peptides (Yi et al., 2017), plant extracts (Abullais Saquib et al., 2021), acidifiers (Pearlin et al., 2020), and plant essential oils (Montassier et al., 2021; Ayalew et al., 2022)—have gained increasing attention. These compounds are valued for their ability to regulate growth performance, immune function, oxidative balance, and intestinal microbial homeostasis in animal models (Roselli et al., 2005). Among these options, probiotics have drawn special interest due to their safety profile, efficacy, and multifunctional benefits in the animal industry (Ningsih et al., 2023).
Probiotics have emerged as promising alternatives to antibiotics in poultry nutrition. These “friendly” bacteria contribute to gut health by enhancing digestion, modulating the immune system, improving intestinal barrier function, and competing against pathogenic microorganisms. Among the various probiotic candidates, species of the Bacillus genus—particularly Bacillus licheniformis—have attracted increasing attention due to their spore-forming capabilities, environmental resilience, and broad-spectrum biological activities. B. licheniformis is “generally recognized as safe” (GRAS) and has demonstrated antimicrobial, antioxidant, and immunomodulatory properties, making it a multifunctional probiotic with diverse applications in poultry production. Recent studies have shown that dietary supplementation with B. licheniformis can significantly enhance growth performance, feed conversion efficiency, egg production, intestinal morphology, and microbial balance in poultry (Pan et.al.2022).
Bacillus licheniformis is a Gram-positive, spore-forming bacterium characterized by high temperature and stress resistance recognized for its probiotic and postbiotic benefits. It produces digestive enzymes such as protease, amylase, lipase, and cellulase, which enhance nutrient utilization. By depleting intestinal oxygen, it fosters anaerobic conditions that promote beneficial bacteria (Lactobacillus, Bifidobacterium) and suppress pathogens (Escherichia coli, Salmonella, Clostridium perfringens). In addition, B. licheniformis secretes bioactive metabolites, including bacteriocins, surfactins, licheniformins, and bacitracin, all of which possess antimicrobial properties (Giri et al. 2019). The bacteriocin, a 42-amino acid peptide (~4.7 kDa), exhibits strong α-helical conformation and acts by disrupting bacterial membranes and inhibiting intracellular processes such as nucleic acid and protein synthesis. These peptides not only suppress pathogens but also enhance host immunity by stimulating neutrophils, macrophages, mast cells, and NK cells, and inducing cytokine and chemokine production. Collectively, B. licheniformis improves feed digestibility, strengthens mucosal barrier function, supports gut microbiota balance, and enhances immune responses, making it a promising candidate for use in both animal nutrition and human health (Shleeva et.al.2023). Moreover, it improves antioxidant status by enhancing the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPX), reduces harmful nitrogen waste (NH₃-N), and boosts microbial crude protein production in ruminants (Jia et al., 2018). Importantly, B. licheniformis supplementation modulates the intestinal microbiota composition and promotes microbial equilibrium, thus maintaining intestinal integrity and performance under disease challenge conditions (Chen and Yu, 2020).
Necrotic enteritis (NE), a major intestinal bacterial disease in poultry caused by Clostridium perfringens, accounts for estimated economic losses exceeding six billion U.S. dollars annually (Wade and Keyburn, 2015). C. perfringens is a Gram-positive, spore-forming anaerobe capable of producing up to 17 toxins (Parish, 1961). Among its toxin-producing variants, types A and C are primarily associated with NE development (Engström et al., 2003). The disease manifests in clinical and subclinical forms. While clinical NE can result in rapid mortality rates up to 50%, subclinical NE (SNE) leads to subtle intestinal injury characterized by reduced appetite, poor nutrient absorption, and depressed growth—all contributing to significant production losses (Timbermont et al., 2011).
NE infections commonly disrupt the intestinal barrier, induce inflammatory responses, and disturb gut microbial balance. Controlling intestinal health, therefore, is an effective preventive strategy against NE in broilers. In the search for sustainable approaches during the post-antibiotic era, probiotics like B. licheniformis have shown substantial potential in alleviating NE symptoms and restoring gut health (Venessa et al., 2016).
Research has demonstrated that B. licheniformis enhances tight junction protein (TJP) and mucin-2 gene expression in poultry, thereby maintaining intestinal integrity and reducing permeability (Wang Y. et al., 2017). Moreover, Bacillus spp. modulate immune responses through upregulation of Toll-like receptors (TLRs), NF-κB signaling, and cytokine synthesis (Rajput et al., 2017). These probiotics also regulate the intestinal microbiota composition, especially under NE challenges, thereby improving host resilience (Lin et al., 2017).
Given its enzymatic activity, antimicrobial peptide production, and regulatory effects on immune and intestinal functions, Bacillus licheniformis represents a viable natural feed additive capable of replacing traditional antibiotics. The present study sought to determine whether B. licheniformis can alleviate subclinical necrotic enteritis (SNE) in broilers as effectively as the antibiotic enramycin, by examining its roles in intestinal barrier maintenance, immune modulation, and gut microbiota balance.
Antimicrobial Peptides (AMPs)
Antimicrobial peptides (AMPs) are a diverse group of small bioactive molecules naturally found across a wide range of organisms. They are essential components of the innate immune system and serve as the first line of defense against a variety of pathogens. AMPs possess broad-spectrum activity against bacteria, fungi, parasites, and viruses, making them crucial in host protection (Huan et al., 2020).
Over the past decades, the rapid rise of antibiotic resistance and growing concerns regarding antibiotic use have driven the exploration and development of AMPs as alternative therapeutic and preventive agents. Their potent antimicrobial properties, coupled with lower rates of resistance development, make them promising candidates for applications in medicine, food preservation, animal husbandry, agriculture, and aquaculture.
Antibacterial Substances Produced by Bacillus licheniformis
The endospore-forming bacterium Bacillus licheniformis is a prolific producer of a wide range of antimicrobial substances, each possessing unique structural and functional characteristics. Interestingly, even when cultured under identical conditions, different B. licheniformis strains synthesize distinct profiles of antibacterial compounds. These variations arise from differences in transcriptional or translational regulation, resulting in strain-specific antimicrobial expression patterns. The molecular masses of these secreted compounds typically range from 1.4 to 20 kDa . The major categories of antimicrobial compounds secreted by B. licheniformis include bacteriocins, licheniformins, bacitracin, and surfactin. (Shleeva et.al.2023).
1. Bacteriocins
Bacteriocins are ribosomally synthesized antimicrobial peptides or proteins that exhibit bactericidal or bacteriostatic activity against closely related bacterial species. B. licheniformis produces multiple types of bacteriocins, typically ranging in molecular weight from 1.4 to 55 kDa, depending on environmental conditions, growth phase, and strain genotype.For instance, B. licheniformis strain B116 secretes a bacteriocin of approximately 4 kDa with potent activity against both Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Listeria monocytogenes, Micrococcus luteus, Bacillus cereus, Escherichia coli, Streptococcus equi, and Salmonella spp. This compound, recovered through ammonium sulfate precipitation, demonstrated strong resistance to heat, acidic, and alkaline conditions. However, enzymatic treatment with pronase completely abolished its antimicrobial activity, and partial inactivation was observed with papain and lipase, indicating the presence of a lipid moiety in its structure (Shleeva et.al.2023).
2. Licheniformins
Licheniformins are lipopeptide antibiotics produced by B. licheniformis, often existing as several closely related variants. The licheniformin lipopeptide produced by B. licheniformis MS3 has a molecular mass of approximately 1.438 kDa. Three principal components—licheniformins A, B, and C—have been identified, all sharing similar amino acid compositions and molecular weights (3.8–4.8 kDa). Despite their structural similarity, these peptides display different degrees of antibacterial potency and toxicity, reflecting subtle biochemical differences in their side-chain structures and lipid modifications (Shleeva et.al.2023).
3. Bacitracin
Bacitracin is a well-known polypeptide antibiotic non-ribosomally synthesized by certain strains of B. subtilis and B. licheniformis. It is composed of 12 amino acids, with four of them—glutamic acid, aspartic acid, phenylalanine, and ornithine—present in their D-isomer forms. The molecular mass of bacitracin is approximately 1.42 kDa. Bacitracin functions by interfering with bacterial cell wall synthesis, making it a clinically important peptide used to inhibit Gram-positive pathogens (Shleeva et.al.2023).
4. Surfactin
B. licheniformis is also capable of producing surfactin and its structural analog, lichenysin—both cyclic lipopeptides with powerful surface-active and antimicrobial properties. The strain B. licheniformis HSN221 was found to secrete nine variants of surfactin and lichenysin when cultured in a medium containing glucose, ammonium chloride, and yeast extract, which were optimal for lipopeptide biosynthesis. The molecular masses of the identified surfactin monomethyl ester homologues were approximately 1.048, 1.049, and 1.063 kDa, as confirmed by ESI-MS analysis. These compounds exhibit potent antimicrobial and emulsifying activity and have potential applications in pharmaceutical, agricultural, and environmental biotechnology (Shleeva et.al.2023).
Mechanism Of Action
1) Competition mechanism:
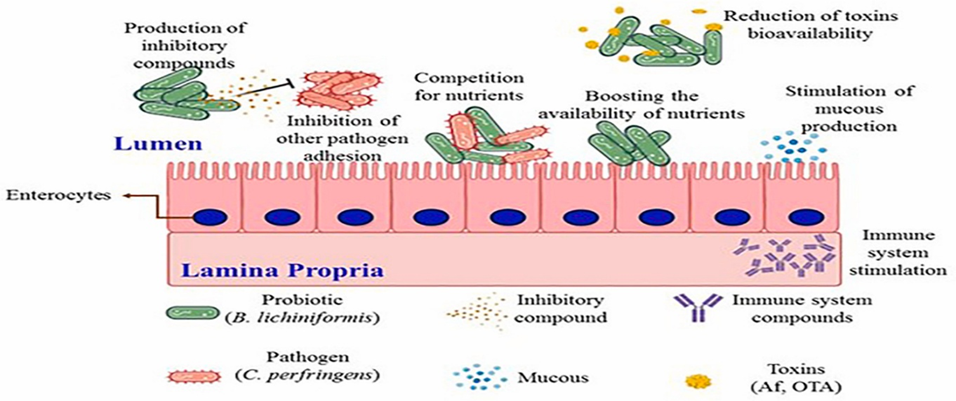
Fig.1. Probiotic Bacillus employ multifactorial competition mechanism to restrict the expansion of pathogens through four pathways.
(1) Bacillus adapt itself in suitable niche against niche-occupying competitors. It approaches the intestinal mucous layer and competitively binds to intestinal epithelial cells and mucous layer components via surface proteins. Thus, the effect of niche occupation by Bacillus expels harmful bacteria from the host intestinal epithelial barrier and reduces pathogen invasion. (2) Competitive utilization of nutrients for Bacillus growth. Bacillus secrete various enzymes to rapidly exploit both the macronutrients and micronutrients in gut environment, resulting in limited availability of nutrition to pathogenic bacteria. (3) Bacillus produce an arsenal of antibacterial metabolites that directly inhibit the growth of pathogens. The metabolites included lipopeptides, bacteriocins, polyketides, and SCFAs are effective against the expansion and invasion of pathogens. (4) Bacillus can consume excess oxygen from gut lumen and host circulation for maintaining the intestinal environment in a state of hypoxia, which drive a dominance of bacteria such as lactate acid bacteria that use fermentation for energy production. (Zhu et.al. 2023).
2) Immunomodulatory activity:
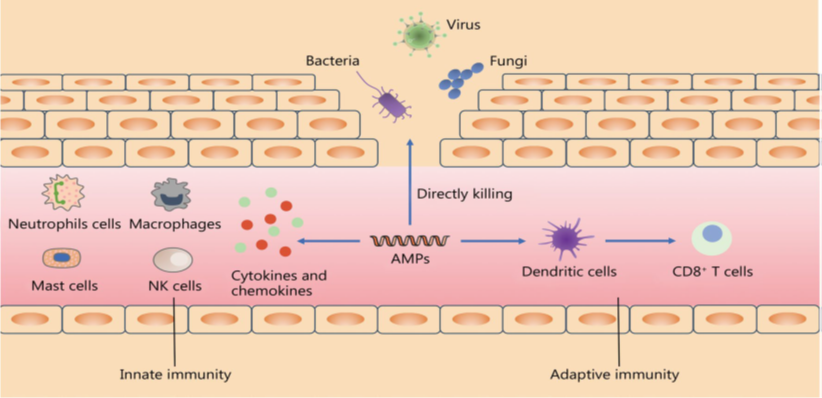
Fig.2. Models of antibacterial mechanisms of AMPs.
Antimicrobial activity and immunomodulatory effects of AMPs, also known as host defence peptides, protect the host from infection. Pathogen invasion triggers a cascade of immunological responses (Fig. 6). Cathelicidins and defensins are mostly produced by neutrophils. The role of AMPs in the immune system is complicated. AMPs regulate the section of cytokines like interleukins; tumour necrosis factors (TNFs), IFNs, and chemokines, as well as the activities of immune cells like dendritic cells (DCs), monocytes, macrophages, mast cells, granulocytes, and lymphocytes, to keep the immune microenvironment in a dynamic balance. AMPs not only destroy invading pathogenic bacteria directly, but they also kill them indirectly by stimulating the immune system. On the one hand, AMPs can activate immune cells in the innate immune system, such as neutrophils, macrophages, mast cells, and NK cells, and trigger the release of cytokines and chemokines to engulf and destroy harmful germs. AMPs, on the other hand, can trigger adaptive immune responses, deliver antigens to T cells via dendritic cells (DCs), and activate cytotoxic T cells to kill pathogenic germs AMPs antimicrobial peptides; NK natural killer. (Babakuliyev et. al.2022).
3) The membrane-disruptive and non-membrane-disruptive antibacterial mechanisms of antimicrobial peptides (AMPs).
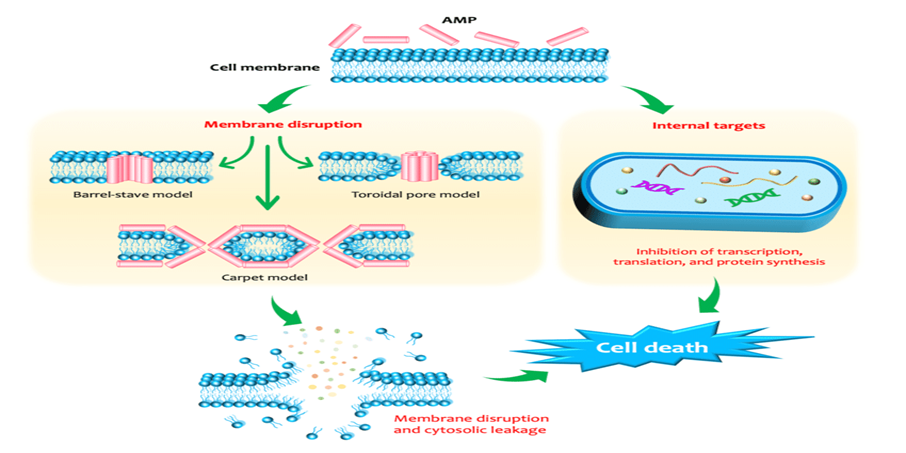
Fig.3. The membrane-disruptive and non-membrane-disruptive antibacterial mechanisms of antimicrobial peptides (AMPs).
In the membrane-disruptive mechanisms, three types of interaction can occur between the membrane and the AMPs, including: (i) barrel-stave model: the peptide monomers form a hydrophilic transmembrane channel by arranging parallelly to the phospholipids of the membrane; (ii) carpet model: the peptides solubilize the membrane into micellar structures; and (iii) toroidal model: the lipid moieties fold inward due to the cascade aggregation of peptide monomers, forming a peptide-and-lipid-lined channel. After AMPs penetrate into the phospholipid membrane, their hydrophobic regions combine with the internal hydrophobic regions of the phospholipid bilayer, while their hydrophilic regions are exposed to the outside. Another bactericidal mechanism is that AMPs penetrate into the cytoplasm and interact with intracellular substances, such as inhibiting DNA, RNA and protein synthesis, inhibiting protein folding, inhibiting enzyme activity and cell wall synthesis, and promoting the release of lyases to destroy cell structures. AMPs antimicrobial peptides. (Le et.al. 2022). The aim of the study to evaluate the effect of PEPIGRO on the performance of commercial broilers reared on deep litter under field conditions.
MATERIALS AND METHODS
Experimental Design and Management
The trial was conducted at Harsh Broiler House -Bilaspur using Vencobb 430 straight run chicks (not sexed at hatchery) in three treatments of around 12000 birds in each treatment. A total of 36000 birds were considered for trial purpose. Feed Formulation used was same for all treatment groups except in T3 where PEPIGRO (Bacillus lincheniformis 3*109) was added at 300 gm per ton feed respectively in all stages. (Table.1). In the study, the energy level was equivalent to the standard requirements of broilers recommended in the Vencobb 430. The trial was carried out over a period of 42 days. The birds were fed ad lib feed and water was available all the time. Care was taken to provide good conditions by adopting strict biosecurity measures. The housing and vaccination procedures were same in both groups.
Table 1. Composition of basal diet for broiler chicks in control group for 3 phases.
| Broiler Feed Formulation (Control) | |||
| Raw Materials | Prestarter | Starter | Finisher |
| Maize | 625.15 | 652.75 | 686.65 |
| HiPro Soya | 335 | 300 | 260 |
| Soya Crude Oil | 6 | 14 | 23 |
| Limestone Powder | 8.5 | 8.5 | 8 |
| Dicalcium Phosphate | 10 | 10 | 8 |
| L Lysine HCI | 2.7 | 2.4 | 2.3 |
| DL Methionine | 3.3 | 3 | 2.7 |
| L Threonine | 1 | 1 | 1 |
| Salt | 2.5 | 2.5 | 2.5 |
| Soda Bi Carb | 1.5 | 1.5 | 1.5 |
| Choline Chloride 60% | 1 | 1 | 1 |
| Organic TM | 0.5 | 0.5 | 0.5 |
| Broiler Vitamin Premix | 0.5 | 0.5 | 0.5 |
| Coccidiostat | 0.5 | 0.5 | 0.5 |
| AGP | 0.05 | 0.05 | 0.05 |
| NSP Enzyme | 0.1 | 0.1 | 0.1 |
| Phytase 5000 | 0.1 | 0.1 | 0.1 |
| Feed Acidifier | 1 | 1 | 1 |
| Toxin Binder | 0.6 | 0.6 | 0.6 |
*The figures are in Kilograms.
The premix provided the following per kilogram of the diet: vitamin A, 6000 IU; vitamin D3, 2500 IU; vitamin B1, 1.75 mg; vitamin B2, 5.5 mg; vitamin B6, 4 mg; vitamin B12, 0.18 mg; vitamin E, 25 mg; vitamin K3, 2.25 mg; Cu, 7.5 mg; Mn, 60 mg; Fe, 75 mg; Zn, 60 mg; Se, 0.15 mg; biotin, 0.14 mg; NaCl, 3.7 g; folic acid, 0.8 mg; pantothenic acid, 12 mg; phytase, 400 U; nicotinic acid, 34 mg; chloride, 350 mg. *Nutrient levels were all calculated values.
Treatment Details-
T1: Control group fed basal diet
T3: Control group fed basal diet + PEPIGRO @300 g PMT
Parameters Studied-
- Body Weight gain was recorded weekly
- Feed Consumption recorded daily and leftover feed was adjusted in the other day quota to know actual intake.
- Mortality was recorded daily
- EEF calculated post harvesting of the flock
- FCR was calculated every week and post harvesting of the flock
Result:
Effect of Pepigro on growth performance parameter in broiler.
Fig.1. Effect of different dietary treatments on Body Weights (g)
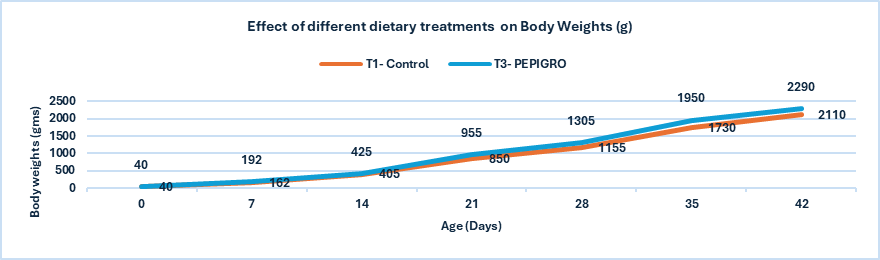
Conclusion: PEPIGRO supplementation at 300g/ton of feed (T3) resulted in a statistically significant 8.18% increase in broiler body weight compared to the control (T1), indicating improved growth performance.
Fig.2. Effect of different dietary treatment on Feed intake (g)
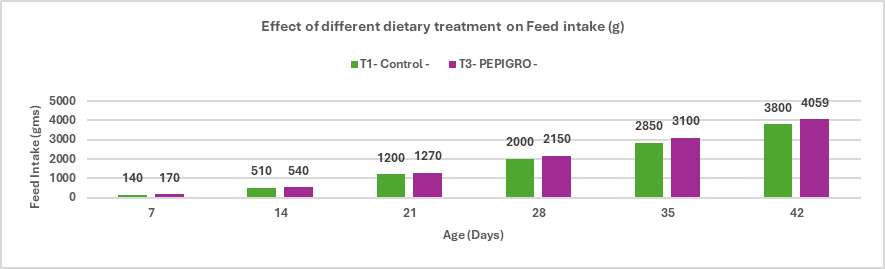
Conclusion: The broiler supplemented with PEPIGRO (T3) at 300g/ ton of feed had a feed intake of 4059 g, which is 6.59% higher than the control group (T1) with 3800 g feed intake. This increase in feed intake indicates that PEPIGRO supplementation positively influenced the birds’ feeding behaviour, likely by enhancing the palatability or nutrient availability of the diet.
Fig.3. Effect of different dietary treatment on Weekly Gain (g)
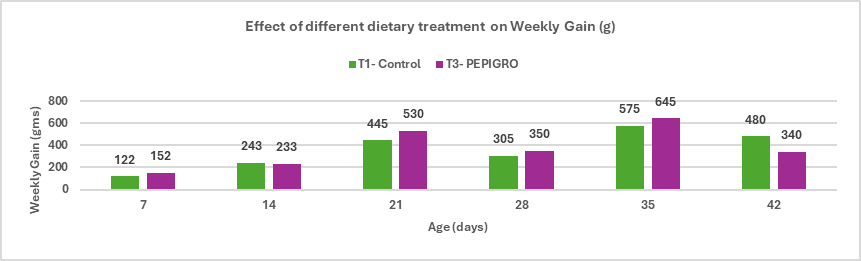
Conclusion: PEPIGRO (T3) supplementation in broiler diet at 300g/ton of feed resulted in the average percentage difference in weekly gain between T1 (Control) is approximately 6.22%. This indicates that PEPIGRO supplementation had a positive overall effect on growth performance, enhancing weight gain efficiency in broiler chickens.
Fig.4. Effect of different dietary treatment on Feed conversion ratio
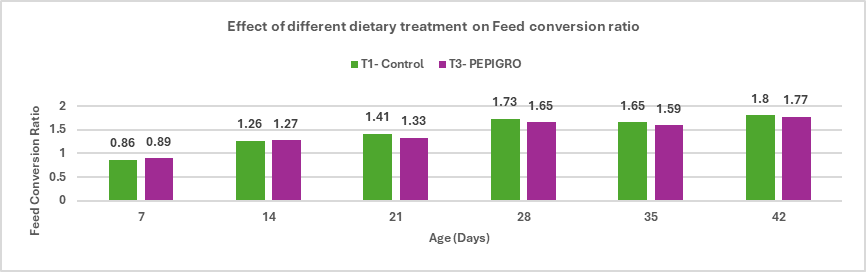
Conclusion: PEPIGRO (T3) supplementation in broiler diet at 300g/ton of feed resulted in a 1.68% improvement in feed conversion ratio (FCR) compared to the control group (T1), indicating enhanced feed efficiency and better growth performance.
Fig.5. Effect of different dietary treatment on Weekly mortality (%)
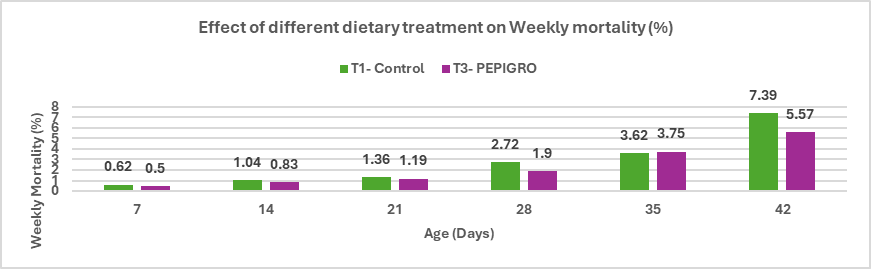
Conclusion: PEPIGRO supplementation at 300g/ton of feed reduced mortality in broiler poultry from 7.39% in the control group to 5.57%, reflecting a 28.08% decrease. This suggests that PEPIGRO may contribute to improved bird health and survivability during the rearing period.
Table 2. Summary of the Report
| Parameters | T1- Control | T3- PEPIGRO | % Difference |
| Body Weight (g) | 2110 | 2290 | 8.18 |
| Feed Intake (g) | 3800 | 4059 | 6.59 |
| FCR | 1.8 | 1.77 | 1.68 |
| CFCR | 1.77 | 1.69 | 4.62 |
| Mortality (%) | 7.39 | 5.57 | 28.08 |
Discussion
The discussion for this article highlights the significant positive effects of PEPIGRO, a Bacillus licheniformis-based probiotic, on the growth performance, feed efficiency, and health status of commercial broilers under field conditions. The 8.18% increase in body weight and 6.59% increase in feed intake, along with improvements in feed conversion ratio (FCR), align well with previous studies showing Bacillus probiotics enhance nutrient digestibility, modulate gut microbial populations, and improve intestinal morphology (Pan et al., 2022; Hung et al., 2019). These effects are especially valuable in the context of rising restrictions on antibiotic growth promoters (Tang et al., 2017), pushing for safer and sustainable alternatives.
The notable 28.08% reduction in mortality observed in this study suggests enhanced resilience of broilers to environmental stressors, likely owing to improved gut barrier integrity and immune modulation. Bacillus licheniformis produces antimicrobial peptides, enzymes, and metabolites such as bacteriocins and surfactins that inhibit pathogens like Clostridium perfringens, a major agent of necrotic enteritis (NE) in poultry (Shleeva et al., 2023; Wade and Keyburn, 2015). PEPIGRO’s capacity to maintain intestinal health and microbial balance may underlie the reduced pathogenic infections and inflammation, consistent with findings that show Bacillus supplementation upregulates tight junction proteins and mucins while enhancing beneficial microbes like Lactobacillus (Chen and Yu, 2020; Wang et al., 2017).
Moreover, the probiotic’s ability to stimulate the host immune system by inducing cytokine production and activating phagocytic cells further supports its protective role in the gut environment (Babakuliyev et al., 2022). This immunomodulatory effect is critical for mitigating subclinical infections and improving overall flock welfare, which translates into better productivity under commercial rearing conditions.
Additionally, PEPIGRO contributes to antioxidant status improvement by elevating enzyme activities such as superoxide dismutase and glutathione peroxidase, reducing oxidative stress that commonly compromises poultry health under heat stress conditions (Jia et al., 2018). This antioxidant benefit complements its antimicrobial and immunomodulatory functions.
In conclusion, this study reinforces the role of Bacillus licheniformis as a multifunctional probiotic that enhances growth performance, feed efficiency, and health in broilers. It offers a sustainable alternative to antibiotics, aligning with global efforts to reduce antibiotic use in animal production. Future studies should explore optimal dosing strategies, combinations with other feed additives, and long-term effects on microbiota composition and immune function to fully harness the benefits of PEPIGRO in commercial poultry systems.
Conclusion-
The trial was conducted in the extreme heat season where average temperature in the surrounding was around 42-45 degree Celsius. The T3 (PEPIGRO) group showed notable improvements compared to the T1 (Control) group. Body weight in T3 (PEPIGRO) increased by 8.18% compared to T1 (Control), indicating better growth performance. Both Feed Conversion Ratio (FCR) and Corrected Feed Conversion Ratio (CFCR) in T3 (PEPIGRO) improved, showing reductions of 1.68% and 4.62%, respectively, compared to T1 (Control), indicating more efficient feed utilization. Additionally, mortality rate in T3 (PEPIGRO) decreased significantly by 28.08% compared to T1 (Control), reflecting better overall health and survival. These results suggest that PEPIGRO supplementation positively impacts growth, feed efficiency, and mortality compared to Control.
References:
B. Rossi, A. Toschi, A. Piva, E. Grilli,Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs Nutr. Res. Rev., 33 (2020), pp. 218-234
B.V. Pearlin, S. Muthuvel, P. Govidasamy, M. Villavan, M. Alagawany, M. RagabFarag, K. Dhama, M. Gopi, Role of acidifiers in livestock nutrition and health: a review J. Anim. Physiol. Anim. Nutr. (Berl.), 104 (2020), pp. 558-569
Babakuliyev, Alisir & Porwal, Prateek & Maiti, Niladri & Singh, Gyan & Khan, Shahbaz & Singh, Gajender & Chanchal, Dilip Kumar. (2022). Design, synthesis, and structural activity relationship of antimicrobial peptides against multi-drug-resistant organisms. International journal of health sciences. 10.53730/ijhs. v6nS2.8211.
E. Montassier, R. Valdés-Mas, E. Batard, N. Zmora, M. Dori-Bachash, J. Suez, E. Elinav, Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner Nat. Microbiol., 6 (2021), pp. 1043-1054
K. Luo, Y. Liu, G. Qin, S. Wang, C. Wei, M. Pan, Z. Guo, Q. Liu, X. Tian ,A comparative study on effects of dietary three strains of lactic acid bacteria on the growth performance, immune responses, disease resistance and intestinal microbiota of Pacific white shrimp, Penaeus vannamei, Fish Shellfish Immunol., 136 (2023), Article 108707
K.L. Tang, N.P. Caffrey, D.B. Nóbrega, S.C. Cork, P.E. Ronksley, H.W. Barkema, A.J. Polachek, H. Ganshorn, N. Sharma, J.D. Kellner, W.A. Ghali, Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis, Lancet Planet Health, 1 (2017), pp. e316-e327.
Le, Mi & Kawada-Matsuo, Miki & Komatsuzawa, Hitoshi. (2022). Efficiency of Antimicrobial Peptides Against Multidrug-Resistant Staphylococcal Pathogens. Frontiers in Microbiology. 13. 10.3389/fmicb.2022.930629.
M. Roselli, A. Finamore, M.S. Britti, P. Bosi, I. Oswald, E. Mengheri, Alternatives to in-feed antibiotics in pigs: evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results,Anim. Res., 54 (2005), pp. 203-218
M. Tian, X. He, Y. Feng, W. Wang, H. Chen, M. Gong, D. Liu, J.L. Clarke, A. van. Eerde Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and counter measures; Antibiotics, 10 (2021), p. 539.
N. Ningsih, A. Respati, D. Astuti, T. Triswanto, L. Purnamayanti, A. Yano, R. Putra, A. Jayanegara, A. Ratriyanto, A. Irawan, Efficacy of Bacillus subtilis to replace in-feed antibiotics of broiler chickens under necrotic enteritis-challenged experiments: a systematic review and meta-analysis, Poult. Sci., 102 (2023), Article 102923.
Pan X, Cai Y, Kong L, Xiao C,Zhu Q and Song Z (2022) Probiotic Effects of Bacillus licheniformis DSM5749 on Growth Performance and Intestinal Microecological Balance of Laying Hens. Front. Nutr. 9:868093. doi: 10.3389/fnut.2022.868093.
Quette Grant, Cyril G. Gay & Hyun S. Lillehoj (2018): Bacillus spp. as directed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry, Avian Pathology, DOI: 10.1080/03079457.2018.1464117
S. Abullais Saquib, N.A. AlQahtani, I. Ahmad, S. Arora, S. Mohammed Asif, M. Ahmed Javali, N. Nisar, Synergistic antibacterial activity of herbal extracts with antibiotics on bacteria responsible for periodontitisJ. Infect. Develop. Count., 15 (2021), pp. 1685-1693
S. Giri, E. Ryu, V. Sukumaran, S.C. Park, Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathogen., 132 (2019), pp. 66-72
Shleeva MO, Kondratieva DA, Kaprelyants AS. Bacillus licheniformis: A Producer of Antimicrobial Substances, including Antimycobacterials, Which Are Feasible for Medical Applications. Pharmaceutics. 2023 Jul 5;15(7):1893. doi: 10.3390/pharmaceutics15071893. PMID: 37514078; PMCID: PMC10383908.
W.H. Organization, Containing Antimicrobial Resistance: Review of the Literature and Report of a WHO Workshop on the Development of a Global Strategy for the Containment of Antimicrobial Resistance. Geneva, Switzerland, 4-5 February 1999 (1999),
Y. Xu, Y. Yu, Y. Shen, Q. Li, J. Lan, Y. Wu, R. Zhang, G. Cao, C. Yang, Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers, Poult. Sci., 100 (2021), Article 101358
Yuchen Huan 1, Qing Kong 1,*, Haijin Mou 1, Huaxi Yi 1, Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields, Front Microbiol. 2020 Oct 16;11:582779. doi: 10.3389/fmicb.2020.582779
Zhu, Jiajia & Chen, Yunsheng & Imre, Kálmán & Arslan Acaroz, Damla & Istanbullugil, Fatih & Fang, Yuwen & Ros, Gaspar & Zhu, Kui & Acaröz, Assoc Prof Dr Ulas. (2023). Mechanisms of probiotic Bacillus against enteric bacterial infections. One Health Advances. 1. 10.1186/s44280-023-00020-0.