Global Poultry Scenario
Salmonella in poultry is one of the major important reasons of production losses through mortality, morbidity and reduced efficiency, with broilers showing the highest prevalence due to intensive farming. In India, where the poultry sector contributes ₹1.3 lakh crore to GDP and supports millions of livelihoods, Salmonella causes losses of up to ₹25,000 crore annually, particularly affecting small-scale farmers (Dhruv et al., 2025).
Predominant serovars such as S. Gallinarum, S. Enteritidis and S. Typhimurium continue to threaten both flock health and food safety, with contamination documented across farms, processing facilities and retail products. According to a global review, the median prevalence of Salmonella was 40.5% in broiler chickens, 30% in raw chicken meat and 40% in eggs and laying hens. Multidrug resistance was found in 97.8% of processing/market studies and 91.1% of farm studies, indicating the presence of resistant reservoirs throughout the supply chain (Castro‑Vargas et al., 2020).
In India, major serovars include S. Gallinarum and S. Pullorum, which cause poultry diseases, alongside foodborne serovars like S. Enteritidis and S. Typhimurium that pose risks to consumers. These infections result in both direct production losses and broader food safety concerns, threatening India’s export growth and domestic confidence. Considering poultry’s role as the most affordable protein source for India’s population and its growing role in international trade, effective Salmonella control through surveillance, vaccination, biosecurity and processing-stage interventions is essential to protect public health, economic sustainability and food security.
Predisposing Factors and Disease Transmission:
Predisposing factors are the factors which are the loop holes or common ignored mistakes on farm which can be harmful as the birds are in continuous dangers because of common pathogens which are always present around them, therefore proper sanitation and biosecurity measures are advised according to the farm type and season to prevent this kind of factor at every step.
Common predisposing factors are:
- Poor hatchery hygiene – contaminated eggs, incubators and hatchery equipment
- Vertical transmission – infected breeder flocks passing bacteria through the egg
- Environmental contamination – dirty litter, contaminated water and feed
- Rodents, wild birds, insects – act as reservoirs and mechanical carriers
- Overcrowding and poor ventilation – stress increases susceptibility
- Inadequate biosecurity – uncontrolled farm access, movement of people/vehicles
- Stress factors – vaccination, transport, feed change, heat stress
- Concurrent infections – immunosuppression from IBD, ND, or any other pre-existing factor.
As these are the factors which provide window to the transmission of Salmonella in poultry farms, therefore proper management of these predisposing factors along with immunization of birds will provide maximum protection from such kind of disease transmission.
Transmission:
Various modes of transmission in poultry are- through vertical transmission from infected breeders to eggs, horizontal transmission via contaminated feed, water, litter, equipment and vectors and last but not least cross-contamination during processing, making it a persistent farm-to-fork threat.
Modes of Transmission:
- Vertical Transmission (Transovarian)
- From infected hens to chicks via eggs
- Important for S. Pullorum and S. Gallinarum
- Horizontal Transmission
- Faecal–oral route: ingestion of contaminated feed, water or litter
- Direct bird-to-bird contact
- Mechanical vectors: rodents, flies, beetles and wild birds
- Farm equipment, boots, vehicles spreading contamination
- During Processing and Marketing
- Cross-contamination during slaughter, defeathering, evisceration
- Contaminated meat and eggs → foodborne infections in humans
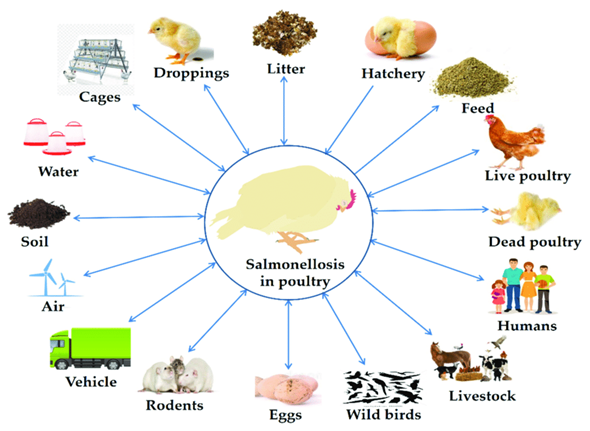
Fig. 1. Modes of transmission of Salmonella
Pathogenesis:
Once ingested by bird, Salmonella colonizes the gastrointestinal tract, particularly the ceca, where its fimbriae, flagella and adhesins enable attachment to the mucosal surface and biofilm formation enhances persistence. It then starts replication in the ceca and establishes a persistent reservoir for faecal shedding. Because the host-adapted serovars like S. Gallinarum and S. Pullorum survive inside macrophages and do not have flagella, they are able to avoid innate immunity and spread throughout the body through the circulation to the liver, spleen, bone marrow and reproductive organs.
In laying hens, colonization of ovaries and oviducts results in trans-ovarian egg contamination, a major food safety concern. Even if the host mounts an innate immune response involving heterophils, macrophages and inflammatory cytokines, followed by adaptive immunity with humoral (IgA, Ig Y) and cell-mediated responses, but clearance is often incomplete, leading to asymptomatic carriers that shed Salmonella intermittently in faeces. Clinical manifestations are most severe in young or immunocompromised birds, resulting in septicaemia, diarrhoea and mortality, while older birds often remain subclinical carriers with minimal lesions.
Pathological changes in acute infections may include enteritis, caecal core formation and necrotic foci in liver and spleen. Thus, the pathogenesis of Salmonella in poultry follows a cycle of ingestion, intestinal colonization, epithelial invasion, intracellular survival, systemic spread, and persistence, with significant consequences for flock health, vertical transmission and zoonotic risk to humans through contaminated poultry products. Hence making it as a dual threat for both poultry health and food safety.
Clinical Signs and Post Mortem lesions:
S. pullorum primarily affects chicks aged 1–3 weeks, causing sudden high mortality, white pasty diarrhoea (pasty butt), weakness, huddling and yolk sac infections, while survivors may develop arthritis and become chronic carriers shedding the organism in eggs and faeces.
While S. gallinarum affects growers and adult birds, producing high fever, anorexia, drooping posture, bronze or darkened combs and wattles, profuse brown-green diarrhoea, swollen necrotic livers and spleens and marked drops in egg production; chronic cases may show milder diarrhoea and shell defects. Key differentiators are age susceptibility (chicks vs adults), faecal colour, presence of arthritis in pullorum disease and severe reproductive impacts in fowl typhoid.
Most common clinical findings:
- Vent paste.
- Ruffled feathers.
- Intestinal haemorrhages.
- Bronze discoloration of liver.
- Elevated white nodular on ventricles.
- Prominent necrotic foci on liver.
- Hepatomegaly.
Post-mortem lesions of Salmonella in poultry are characteristic and vary with age and disease type. In Fowl Typhoid (caused by S. Gallinarum), the liver becomes enlarged, friable and bronze in colour, often seen with necrotic foci, while the spleen and kidneys are swollen and congested. The heart shows petechial haemorrhages and intestines display catarrhal to haemorrhagic enteritis, sometimes with button-like ulcers in chronic cases. In Pullorum Disease (caused by S. Pullorum), chicks show white necrotic foci in the liver, spleen and lungs, caecal cores, enlarged pale kidneys and unabsorbed yolk sacs. In older birds, lesions include caseous oophoritis, salpingitis and peritonitis. These lesions highlight the septicaemic nature of Salmonella, leading to systemic organ damage and reproductive losses in poultry.
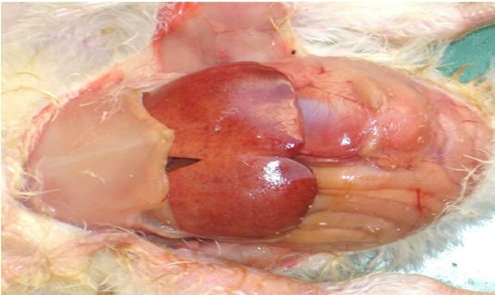
Fig 2. Necrotic foci on liver, in chicken

Fig. 3: Vent paste, diarrhoea and huddling in chicks.
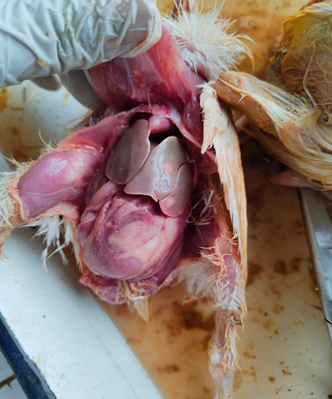
Fig. 4: Bronze discolouration of liver

Fig. 5: Congested ovarian follicles
Diagnosis:
Although many carriers exhibit no symptoms, the first step in diagnosing Salmonella in poultry is to look for clinical indicators and post mortem lesions.
Diagnosis should be based on:
- Flock history
- Clinical signs
- PM lesions
- Laboratory confirmation
Affected birds often show depression, ruffled feathers, white diarrhea with pasted vents, reduced egg production, and high mortality, especially in chicks. Post-mortem examination typically reveals hepatomegaly with bronze discoloration and necrotic foci, splenomegaly, nephritis, cecal cores, and yolk sacculitis. Definitive diagnosis requires bacteriological culture from liver, spleen, bile, or cecal contents using enrichment broths and selective agars, supported by biochemical tests. Rapid plate agglutination tests are commonly used for flock screening, while molecular methods like PCR and ELISA provide sensitive and specific detection of Salmonella. Also, NPIP protocols detail validated sampling methods (drag/manure swabs, cloacal swabs, hatchery debris), culture workflows and confirmatory testing through biochemical and serological ID (9 C.F.R.) This integrated algorithm ensures early detection, regulatory compliance and reduced Salmonella transmission across poultry systems.
Treatment and Drug Resistance:
Antibiotics are primarily used to treat Salmonella infections in chickens in order to lower mortality and clinical illness; because carrier states persist, total eradication of the bacteria is rarely possible. Among commonly used medications include aminoglycosides, sulphonamides with trimethoprim, tetracyclines and fluoroquinolones. Probiotics, vitamins and electrolytes used in supportive therapy aid in gut health and recuperation. However, Salmonella has developed antimicrobial resistance (AMR) as a result of the overuse and abuse of antibiotics in chicken farming.Alarmingly, many isolates demonstrate multi-drug resistance, complicating treatment options for both poultry and human infections (Das et al., 2023)
These challenges create major public health risks and economic burdens, underscoring the urgent need for integrated control strategies. Vaccination of breeder and layer flocks—using live-attenuated and killed vaccines—has proven highly effective in reducing colonization, shedding, vertical transmission and cross-serovar infections, thereby lowering environmental contamination. Therefore, effective control of Salmonella in poultry with combined use of strict biosecurity and strategic vaccination, can fully prevent infection with less or no use of excess antibiotics. Even effective vaccination can reduce mortality, also egg and meat contamination and decreases reliance on antibiotics.
Prevention and control:
Salmonella control in poultry relies on a multifaceted strategy that integrates strict biosecurity, environmental hygiene, hatchery and egg sanitation, prudent antimicrobial use and continuous monitoring, with vaccination serving as a cornerstone. Rigorous access control, pest management, litter and water sanitation and hatchery hygiene help minimize pathogen entry and spread, while surveillance using culture, serology and molecular diagnostics ensures early detection and program adjustment. Antimicrobial stewardship reduces resistance risks and alternatives like probiotics support gut health. Above all, live and killed vaccines, tailored to local serovars, not only reduce intestinal colonization and vertical transmission but also complement other measures to sustainably lower Salmonella prevalence, protect food safety and safeguard public health.
Stallen South Asia Pvt. Ltd. Is offering unique live vaccine BIO-VAC SGPN 695 against fowl typhoid and Salmonella enteritidis.
Key Features of BIO-VAC SGP 695:
- Live-attenuated dual‐serovar vaccine: BIO-VAC SGP 695 uses a stable, non-reverting Salmonella gallinarum/pullorum strain to protect pullets against both fowl typhoid and pullorum disease, while also reducing Salmonella enteritidis colonization in the gut.
- Oral, water-based delivery: The freeze-dried vaccine is easily reconstituted and administered via drinking water, ensuring uniform uptake across large flocks without injections and stress.
- Rapid, durable immunity: Two doses elicit strong protective responses leading to lower mortality, milder clinical signs and stabilized egg production.
- Cost-effective and field-stable: Bulk reconstitution lowers per-dose handling and labour costs, and the strain remains apathogenic under farm conditions without interfering with other vaccination programs.
| Feature | BIO-VAC SGP 695 | SG 9R |
| Targeted infections | Salmonella gallinarum, Salmonella pullorum, Salmonella Enteritidis | Primarily Salmonella gallinarum (Fowl Typhoid) |
| Characterisation | Stable attenuation, non-reverting; safe in pullets | Possible reversion |
| Administration | Oral via drinking water (no handling stress, flock-wide coverage) | Injection (subcutaneous/intramuscular) labour intensive, stressful |
| Vaccination program | 2-dose pullet program: 6–8 weeks & 16–18 weeks | Multiple injections required; less practical in large flocks |
| Efficacy | Strong systemic & mucosal immunity; reduces colonization, shedding and mortality | Inconsistent field efficacy; does not prevent shedding fully |
| Field advantages | Easy mass application, safer profile, compatible with biosecurity programs | Higher labour cost, handling stress, potential post-vaccination reactions |
Table No. 1.: Comparison of SGP 695 and SG 9R
- One dose of vaccine contains: Cultures of S. gallinarum/pullorum attenuated strain SGP 695 AV: min. 2×108 CFU.
- Able to stimulate a rapid and long-lasting immune response.
Field trial results of BIO VAC SGP 695:
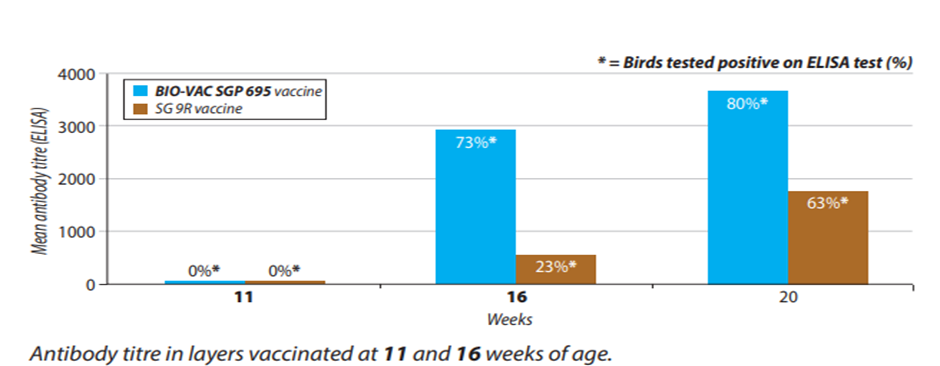
Fig. 3: ELISA report of BIO-VAC SGP 695 and SG 9R vaccine
- Difference between SET-VAC and BIO-VAC SGP 695:
- Live attenuated vaccines:
- Are typically used in layer flocks.
- Induction of both humoral immunity (B cells) and cell-mediated immunity (T-cells).
- Inactivated vaccines:
- More commonly used in breeder flocks.
- Induce strong antibody responses.
| SET VAC | BIO-VAC SGP 695 | |
| Type | Inactivated | Live |
| Strain | S. enteritidis and S. typhimurium | S.gallinarum, S.pullorum, S. enteritidis |
| Administration | Subcutaneous injection | Oral (via drinking water) |
| Vaccination Program | Initial dose at 6-8 weeks, second at 14-16 weeks. | Initial dose at 6-8 weeks, second at 16-18 weeks. Early dose if early infection history. |
- ADMINISTRATION AND DOSAGE:
- In drinking water not before the age of 7 days.
- For pullet vaccination at 6-8 weeks of age followed by a second vaccination at 16-18 weeks of age.
- Additional vaccination: 7-10 days old if severe infection or history of early infection, Ideal for pullets before ovulation. Safe for laying hens
- NOTE:
- Make sure that there are no antiseptic or disinfectant agents in the water used for dilution.
- Make sure that all birds drink the vaccine suspension within 2 hours.
- After dissolving in water, it should be used within 2 hours.
- STORAGE:
- Store in the refrigerator between +2° C and +8° C.
- DOSES PER PACK:
- 1000 doses per vial.
References:
- 9 C.F.R. 145.14 (2025). https://www.ecfr.gov/current/title-9/chapter-I/subchapter-G/part-145/subpart-A/section-145.14
- Castro‑Vargas, R. E., Herrera‑Sánchez M. P., Rodríguez‑Hernández R. and Rondón‑Barragán I. S. (2020). Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Veterinary World, 13(10): 2070–2084. https://doi.org/10.14202/vetworld.2020.2070-2084
- Das, A. et al. (2023). Antimicrobial resistance in Salmonella isolates from Indian poultry: a systematic review. Frontiers in Microbiology, 14: 10920579.
- Gaur D., Kukreti D., Ahuja S., Kumar S. and Singh A. (2025). Poultry diseases rising concern in India. International Research Journal of Modernization in Engineering Technology and Science, 7(4): 8510–8513. https://doi.org/10.56726/IRJMETS74074.




